What needs to be on the cosmetic product label?

To guarantee consumer safety, cosmetic products must include in their packaging all the information necessary for their safe use. Specifically, according to Regulation (EC) No 1223/2009, cosmetic products shall only be made available on the market where their container and packaging bear the following information in indelible, easily legible, and visible lettering:
- The name and the address of the responsible person. When several addresses are indicated, the one where the responsible person makes readily available the Product Information File (PIF) shall be highlighted.
- The country of origin in the case of imported cosmetic products.
- The nominal content at the time of packaging, given by weight or by volume.
- The indication of the date of minimum durability (DMD) and/or the period after opening (PaO).
- Precautions to be observed in use and any special precautionary information on cosmetic products for professional use.
- The batch number of manufacture or the reference for identifying the cosmetic product.
- The function of the cosmetic product unless it is clear from its presentation.
- Alist of ingredients, preceded by the term “ingredients” and established in descending order of weight of the ingredients at the time they are added to the cosmetic product. Ingredients in concentrations of less than 1% may be listed in any order after those in concentrations of more than 1%. Colorants other than colorants intended to colour the hair may be listed in any order after the other cosmetic ingredients.
As far as the ingredient list is concerned, there are still other aspects to consider that deserve special mention:
- Ingredients shall be expressed by using the common ingredient name set out in the Glossary of common ingredient names.
- Perfume and aromatic compositions and their raw materials shall be referred to by the terms “parfum” or “aroma”. Moreover, the presence of substances, the mention of which is required under the column ‘Other’ in Annex III to Regulation (EC) No 1223/2009, must be indicated in the list of ingredients in addition to these terms.
- All ingredients present in the form of nanomaterials shall also be clearly indicated by using the word “nano” in brackets following the name of the ingredient.
- For decorative cosmetic products marketed in several colour shades, all colorants other than colorants intended to colour the hair used in the range may be listed, provided that the words “may contain” or the symbol “+/-” are added. The CI (Colour Index) nomenclature shall be used, where applicable.
The Cosmetic Regulation also states that where it is impossible for practical reasons to label the list of ingredients, as well as the precautions to be observed in use, this information shall be mentioned on an enclosed or attached leaflet, label, tape, tag, or card, which shall be referred by abbreviated information or the following symbol:
What do we need to know more?
In addition, some related horizontal labelling requirements may also apply. The Aerosol Dispensers Directive 75/324/EEC and its adaptations to technical progress (94/1/EC and 2008/47/EC), establish the rules for aerosols with a capacity of more than 50 ml, including specific requirements related to flammability and pressure hazard related to aerosols dispensers and requiring the apposition of the so-called “inverted-epsilon” symbol certifying conformity with this Directive:
Moreover, sunscreen products may also be subject to additional label requirements specified on the Recommendation on the efficacy of sunscreen products and the claims made relating thereto. Although this is not a directive or a regulation, this document aims to standardise the way sunscreen products are labelled in the European Union (EU) and addresses the aspects relating to claims made for sunscreen products and the efficacy of such products, namely how the labelling of sunscreen products can be kept simple and comprehensible to assist the consumer in choosing the appropriate product. Among other aspects, this document establishes that:
- The efficacy of sunscreen products should be indicated on the label by reference to categories such as “low”, “medium”, “high” and “very high” that should be labelled at least as prominently as the sun protection factor (SPF).
- Sunscreen products should display warnings indicating that they do not provide 100 % protection and advice on precautions to be observed in addition to their use.
- Sunscreen products should carry instructions for use that will ensure that the claim made for the effectiveness of the product can be achieved.
- Sunscreen products should carry instructions for use to ensure that a sufficient quantity is applied on the skin to achieve the effectiveness claimed for the product.
In order to indicate that a sunscreen product offers a minimum UVA protection as endorsed in the Recommendation, Cosmetics Europe has issued a standardised UVA-label, consisting of the letters “UVA”, printed in a simple circular shape:
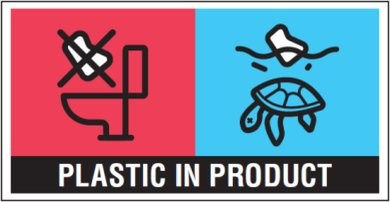
More recently, with the adoption of the Single-Use Plastics Directive (EU) 2019/904 on the reduction of the impact of certain plastic products on the environment and the new Commission Implementing Regulation (EU) 2020/2151 laying down rules on harmonised marking specifications on single-use plastic products listed in Part D of the Annex to the Directive, new labelling provisions are also in place for wet wipes (i.e., pre-wetted personal care and domestic wipes). Specifically, since 3 July 2021, packaging of wet wipes, with the surface area of 10 cm2 or more, shall bear the following printed marking:
What should we also be aware of?
Although not mandatory, other symbols and labelling policies may also apply to cosmetic products, in order to standardise the way information is presented and facilitate the sale of products throughout the EU market. For instance, it is recommended to affix the ℮-mark next to the nominal quantity of the product as it shows that the product complies with EU rules on the indication of the volume or weight and the measuring methods that you must use as a seller of pre-packaged products:
Additionally, at a time when sustainability is truly the watchword, packaging waste labelling has also been one of the most debated topics and although there is still no harmonisation, some practices have been adopted. The “Green Dot” financing symbol(see below on the left), for example, is widely used across the EU, being mandatory in Spain and Cyprus, and means that for such packaging a financial contribution has been paid to a qualified national packaging recovery organization; it does not have, however, any environmental or recycling meaning. Specific national requirements are also in effect in some EU member states such as Italy, which introduced, in 2020, a mandatory environmental labelling on the packaging of products, and France, where the “Triman” symbol introduced (see below on the right) by the Decree 2014-15733, must be mandatorily displayed on the labels of recyclable products, including cosmetics, since 1 January 2022.

How we help
As can be seen, checking the labelling requirements that your cosmetic products must meet can be a challenging task, demanding attention to all applicable requirements and effective communication for the prevention of non-conformities. Cosmedesk offers a labelling review functionality, which in addition to generating the list of ingredients of your product, provides a checklist with the general provisions, easily editable and adaptable to your product type. At the end, you can generate a labelling review report that you can share with your team, customers, or partners.
References
Cosmetics Europe: Guidelines on Cosmetic Product labelling, 2011
European Commission. Cosmetic products – specific topics: Sunscreen products
European Commission. Labels and markings: e-mark
Packaging Recovery Organisation Europe (PRO Europe). The Green Dot Trademark
![[Logo Cosmedesk]](/media/ljbbhnk4/logo-cosmedesk.svg)